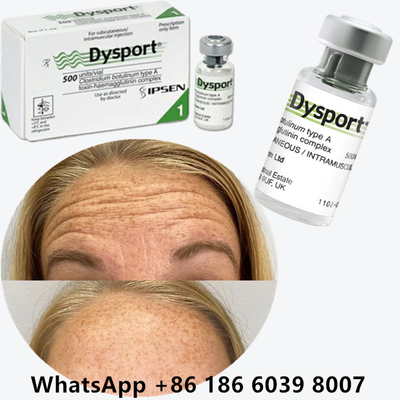
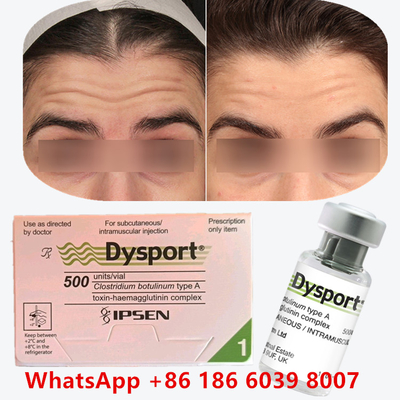
Botulinum Toxin Dysport Botox Clostridium Botulinum Type A For Tear Troughs
Product Details:
| Place of Origin: | China |
| Brand Name: | Dysport |
| Certification: | NMPA FDA |
| Model Number: | Dysport |
Payment & Shipping Terms:
| Minimum Order Quantity: | 1 box |
|---|---|
| Price: | $35/box |
| Packaging Details: | 1vials/box |
| Delivery Time: | 3-5 work days |
| Payment Terms: | Ali, Paypal, T/T, Western Union, MoneyGram |
| Supply Ability: | 100000pcs per month |
|
Detail Information |
|||
| Product: | Botulinum Toxin Dysport Botox | Material: | Clostridium Botulinum Type A |
|---|---|---|---|
| Treatment: | For Tear Troughs | Another Name: | Dysport Injection |
| Type: | Botulinum Toxin Type A | Color Of: | White |
| Contraindications: | Pregnancy, Breastfeeding, Neuromuscular Disorders | Treating Areas: | Facial Wrinkles And Folds |
| Injection: | Injection For Facial | Application: | Face |
| Feature: | Firming,Anti-Wrinkle,Anti-aging,Effect | Shipping: | With Ice Bag |
| Term Of Validity: | 6 Months | Storage: | Refrigerated |
| Duration: | 3-6 Months | Odor: | Odorless |
| Highlight: | Botox Clostridium Botulinum Type A,Tear Troughs Clostridium Botulinum Type A |
||
Product Description
Botulinum Toxin Dysport Botox Clostridium Botulinum Type A For Tear Troughs
Post-Treatment Care
After receiving Dysport, follow post-treatment care instructions closely. Avoid strenuous activities for at least 24 hours. This precaution helps prevent complications and ensures optimal results.
Your doctor may advise against lying down for several hours post-treatment. Staying upright allows the product to settle properly in the targeted area.
![]()
![]()
![]()
![]()
Specifications:
| Product | Botulinum Toxin Dysport Botox |
| Material | Clostridium botulinum type A |
| Treatment | For Tear Troughs |
| Another name | Dysport Injection |
| Type | Botulinum toxin Type A |
| Color Of | White |
| Contraindications | Pregnancy, Breastfeeding, Neuromuscular Disorders |
| Treating Areas | Facial wrinkles and folds |
| Injection | Injection For Facial |
| Application | Face |
| Feature | Firming,Anti-Wrinkle,Anti-aging,Effect |
| Shipping | with Ice Bag |
| Term Of Validity | 6 months |
| Storage | Refrigerated |
| Duration | 3-6 months |
| Odor | Odorless |
Discuss with Healthcare Provider
Share Medications
Patients should share all current medications with their healthcare provider. This includes prescription medicines, nonprescription drugs, and any supplements. Each medication can interact with Dysport. Even common over-the-counter medicines may affect treatment.
Keeping an updated list helps the doctor make informed decisions. This list should include dosages and frequency of use. Disclosing this information ensures safety during treatment.
History of Difficulties
Discussing any history of swallowing or breathing problems is crucial. Patients with such issues may face increased risks when using Dysport. The botulinum toxin can cause muscle weakness, which may worsen these conditions.
Healthcare providers need to know these details. They can assess the risk and adjust treatment plans accordingly. Open communication about past difficulties leads to safer outcomes.
![]()
Post-Treatment Care
After receiving Dysport, follow post-treatment care instructions closely. Avoid strenuous activities for at least 24 hours. This precaution helps prevent complications and ensures optimal results.
Your doctor may advise against lying down for several hours post-treatment. Staying upright allows the product to settle properly in the targeted area.
Before & After:
![]()
Packing and Shipping:
![]()
![]()
![]()