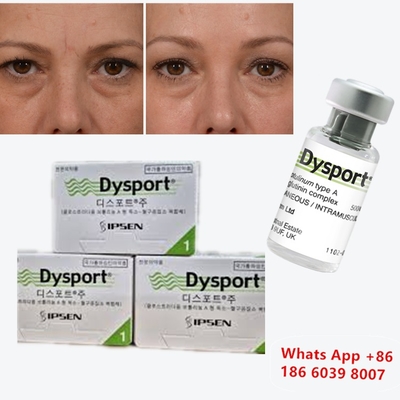
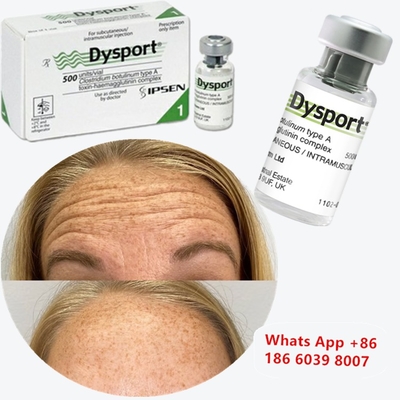
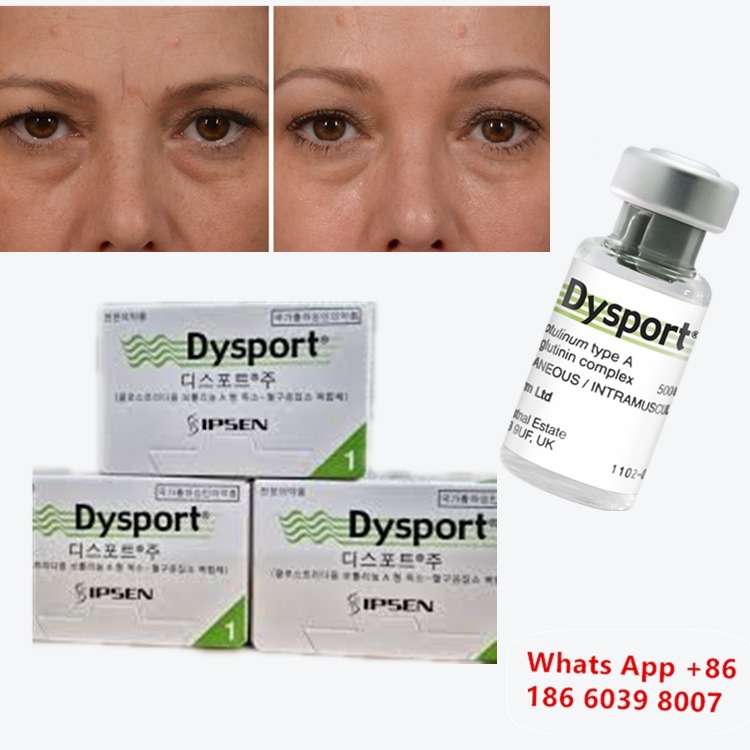
Botulinum Toxin Dysport Clostridium Botulinum Type A For Nose Correction
Product Details:
| Place of Origin: | China |
| Brand Name: | Dysport |
| Certification: | NMPA FDA |
| Model Number: | Dysport |
Payment & Shipping Terms:
| Minimum Order Quantity: | 1 box |
|---|---|
| Price: | $35/box |
| Packaging Details: | 1vials/box |
| Delivery Time: | 3-5 work days |
| Payment Terms: | Ali, Paypal, T/T, Western Union, MoneyGram |
| Supply Ability: | 100000pcs per month |
|
Detail Information |
|||
| Product: | Botulinum Toxin Dysport | Material: | Clostridium Botulinum Type A |
|---|---|---|---|
| Treatment: | For Nose Correction | Another Name: | Dysport Injection |
| Type: | Botulinum Toxin Type A | Color Of: | White |
| Contraindications: | Pregnancy, Breastfeeding, Neuromuscular Disorders | Treating Areas: | Facial Wrinkles And Folds |
| Injection: | Injection For Facial | Application: | Face |
| Feature: | Firming,Anti-Wrinkle,Anti-aging,Effect | Shipping: | With Ice Bag |
| Term Of Validity: | 6 Months | Storage: | Refrigerated |
| Duration: | 3-6 Months | Odor: | Odorless |
| Highlight: | Dysport botulinum toxin for nose,Botulinum toxin type A nose correction,Dysport Clostridium botulinum treatment |
||
Product Description
Botulinum Toxin Dysport Clostridium Botulinum Type A For Nose Correction
Importance of Breastfeeding Considerations
For breastfeeding mothers, discussing plans with the healthcare provider is vital. The effects of Dysport on breast milk are not fully understood. Sharing this concern allows the doctor to provide guidance on safety.
Breastfeeding mothers must weigh the benefits against potential risks. A healthcare provider can help navigate this decision effectively.
Adjusting Treatment Plans
Changes in health status or new medications require ongoing dialogue with the healthcare provider. If a patient experiences side effects or other complications, they should inform their doctor immediately.
Regular updates help maintain a safe treatment environment. Adjustments in dosage or alternative treatments may be necessary based on individual responses.
Life Quality Impact
Dysport aims to improve quality of life for many patients experiencing muscle-related issues. However, open discussions ensure that it contributes positively without adverse effects.
![]()
![]()
![]()
![]()
Specifications:
| Product | Botulinum Toxin Dysport |
| Material | Clostridium botulinum type A |
| Treatment | For Nose Correction |
| Another name | Dysport Injection |
| Type | Botulinum toxin Type A |
| Color Of | White |
| Contraindications | Pregnancy, Breastfeeding, Neuromuscular Disorders |
| Treating Areas | Facial wrinkles and folds |
| Injection | Injection For Facial |
| Application | Face |
| Feature | Firming,Anti-Wrinkle,Anti-aging,Effect |
| Shipping | with Ice Bag |
| Term Of Validity | 6 months |
| Storage | Refrigerated |
| Duration | 3-6 months |
| Odor | Odorless |
Discuss with Healthcare Provider
Share Medications
Patients should share all current medications with their healthcare provider. This includes prescription medicines, nonprescription drugs, and any supplements. Each medication can interact with Dysport. Even common over-the-counter medicines may affect treatment.
Keeping an updated list helps the doctor make informed decisions. This list should include dosages and frequency of use. Disclosing this information ensures safety during treatment.
History of Difficulties
Discussing any history of swallowing or breathing problems is crucial. Patients with such issues may face increased risks when using Dysport. The botulinum toxin can cause muscle weakness, which may worsen these conditions.
Healthcare providers need to know these details. They can assess the risk and adjust treatment plans accordingly. Open communication about past difficulties leads to safer outcomes.
Past Reactions
Informing the doctor about any past reactions to botulinum toxin products is essential. Some individuals may have allergies to these substances. Allergic reactions can range from mild to severe.
Patients should also disclose other allergies, including skin reactions or infections. This information helps the healthcare provider determine if Dysport is appropriate. Understanding a patient’s medical history aids in tailoring treatment.
![]()
Post-Treatment Care
After receiving Dysport, follow post-treatment care instructions closely. Avoid strenuous activities for at least 24 hours. This precaution helps prevent complications and ensures optimal results.
Your doctor may advise against lying down for several hours post-treatment. Staying upright allows the product to settle properly in the targeted area.
Before & After:
![]()
Packing and Shipping:
![]()
![]()
![]()